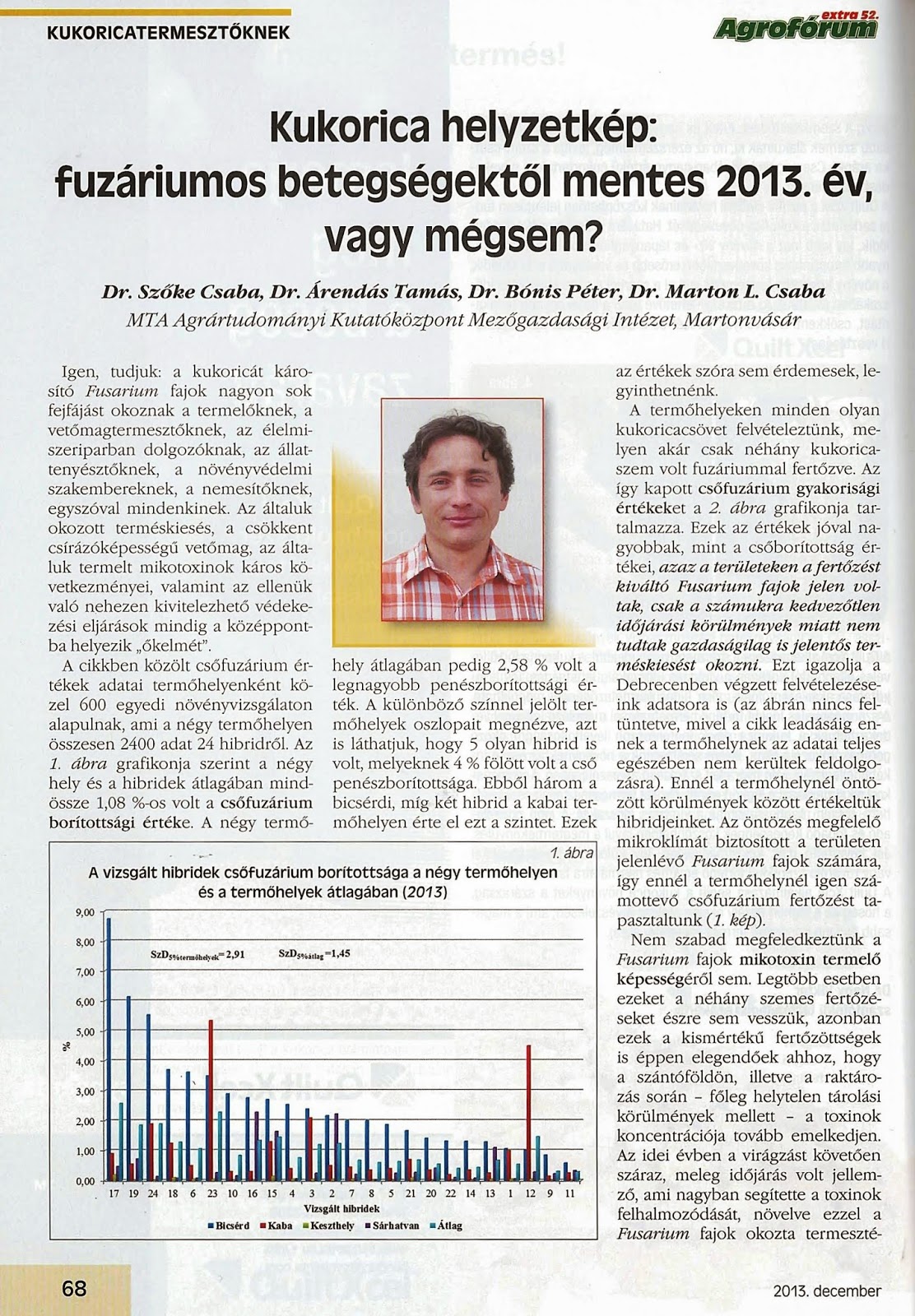
In
the plant protection of maize, the integrated protection has to be applied.
Thus, the selection of the growing area, that of the hybrid and the
professional application of the agrotechnical elements (crop rotation, tillage,
sowing, etc.) are important. In the case of adequate crop rotation, the most
important field of the protection of maize is weed control. With crop rotation,
we can successfully protect against either the animal pests (mainly the
American corn rootworm) or the plant diseases (mainly Fusarium).
The diseases of maize and the protection
against them
Currently,
in the domestic maize production, a relatively low number of pathogens can be
observed, their damages differ, primarily depends on the cropyear.
The
principal maize diseases considered are the following:
·
Setosphaeria
turcica (Northern Leaf Blight);
·
Sphacelotheca
reiliana (Head smut);
·
Ustilago
maydis (Common smut);
·
Colletotrichum
graminicola (anthracnose);
·
Sclerophthora
macrospora (downy mildew);
·
Nigrospora
oryzae
·
Maize dwarf mosaic virus
·
Fusarium spp. (Cob
and stem rot);
Northern Leaf Blight, caused by
Setosphaeria turcica /anamorph Exserohilum
turcicum/ (Figure 1). It is a serious fungal disease prevalent in cooler
climates and tropical highlands wherever maize is grown. It is characterized by
large elliptical shaped necrotic lesions that develop on the leaves due to the
polyketide metabolite monocerin.
Figure 1 Setosphaeria turcica (Source: Takamiya and Sendo, 2000)
Head smut (Figure 2/a) is a disease of maize found in the major maize growing areas of Europe and the United States. The causal agent is Sphacelotheca reiliana, a fungus that attacks corn plants sporadically throughout the field, invading some plants, but leaving others unaffected. The pathogen cannot be transmitted from one plant to the other in the field and affected plants have no grain development. Even with a relatively low percentage of infection in the fields (10%), yield reduction can be significant. Infection rates up to 80% have been reported. Once the infection occurs, there are no effective treatments for reducing or eliminating the damage on affected plants.
Common smut of maize, caused by Ustilago
maydis (Figure 2/b), is easily identified by tumor-like galls that form on
actively growing host tissues and contain masses of dark, sooty teliospores.
Throughout most of the world, common smut is considered to be a troublesome
disease of maize. In addition to the practical significance of causing a
prevalent disease and being an edible fungus,
U. maydis also has been used as a model organism to study a variety of
interesting biological phenomena.
Colletotrichum graminicola (Figure 3)
causes anthracnose stalk rot and leaf blight of maize. C. graminicola is a major cause of stalk rot disease, one of the
most economically important diseases of maize. Industry estimates are that stalk
rots causes maize yield losses in the range of 6% annually. C. graminicola also causes a leaf blight
that is becoming increasingly important, particularly in the tropics and
subtropics. C. graminicola is among
the best characterized and most tractable of the Colletotrichum fungi. It is one of very few in which sexual crosses
can be made (the teleomorph is Glomerella
graminicola); it is easily cultured and stored; transformation and gene
disruption are routine; and pathogenicity assays are straightforward. Maize,
its host, is a classical genetic model as well as an important crop plant.
Sclerophthora macrospora (Figure 4)
causes systemic infection. Symptoms vary greatly with the time of infection and
the degree of colonization. Plants show excessive tillering and narrow
chlorotic leaves, but the most characteristic symptom, usually known as crazy
top, consists of partial or complete proliferation of the tassel, which is
finally transformed into a mass of leafy structures. Phyllody may also occur in
the ears. Infections start from the resting oospores which are frequently
formed on perennial grasses. Seed-borne mycelium can also be a source of
infection. The disease usually occurs only in restricted, low-lying,
inadequately drained areas or after severe floods.
Figure 4 Sclerophthora macrospora (Source: Boehm, 2000)
Nigrospora oryzae (Figure 5)
causes systemic infection. Causal agent infects maize seedlings and adult
plants. The lower part of pedicle turns brown and softens; plants lodge and
rot. In the following vegetation period, the first symptoms of the Nigrospora
Ear Rot are shredding of pedicle and lower part of shanks, black spore masses
are scattered in shredded pith of the cob and on the kernel tips. Later black
abundant scurf develops on cob and on reproductive buds in leaf sheaths. Under
strong disease development ears remain undeveloped and lightweight. Affected
kernels are dull, slightly grayish, and undeveloped.
Maize dwarf mosaic virus (Figure 6).
The infected plants have, at the base of the leaves, a light green – yellow
striped pattern, which develop along the leaves. All of the leaves are infected
on the plant, the ear covering ones too. The light green patterns often occur
around dark green islands on the leaves. At higher temperatures, the symptoms
are lighter and tend to disappear, but the youngest leaves are very light
green. The plant grows slowly, slightly stunted. Earlier infection may cause
stalk and root rot, and the infected young plant may die. The symptoms, except
for depressed growth, are very similar to other viruses or to physiological
yellowing. The viruses always occur in the field sporadically, the
physiological problems in smaller-bigger spots. Maize viruses often occur
together, “pure” MDMV infection is extraordinarily rare. Consequently, that nearly
all maize viruses can survive the winter in the Johnson grass, and the vectors
transport them usually together.
Figure 6 Maize dwarf mosaic virus (Source: Agroatlas, 2003)
The
most considerable damages are caused by Fusarium
spp (FigureF. graminearum, F. culmorum,
F. verticillioides and F. subglutinans. They can be seed-borne but, in general, survival is
on plant debris. Air-borne spores are the main source of inoculum. Symptoms
appear at ripening. The pathogens attack the stem from the soil through the
roots or lower stem internodes. After a severe attack, the foot of the stem
rots completely and the stem dies. Partly severed ears may hang down after
being attacked, which can obstruct the harvesting of the maize plants and cause
severe yield reduction. Depending on the year, the pathogen may cause yield
losses of 6–35%, but the qualitative damage caused by the mycotoxins produced
by these pathogens is a much bigger problem. These secondary metabolic products
not only result in a reduction in the nutritional value of the feed, but are
also absorbed by the digestive system, accumulating in the tissues and causing
severe abnormalities in the digestive and sexual organs. They also have an
indirect effect, since the consumption of animal products contaminated with
mycotoxins represents a potential risk for humans. The most effective way of
combating these pathogens is through maize resistance breeding. The mechanism
of resistance to individual Fusarium species has not yet been completely clarified.


Figure 7/a-b Fusarium ear and stalk rot (Source: Szőke, 2011)
The animal pests of maize and the protection
against them
Maize
stocks can be threatened by animal pests from sowing to harvest. Birds and
mammals cause considerable damages only occasionally. The significance of
insect pests are much greater, among them the most important ones are the
following:
·
Soil insects (Agriotes
spp., Melolontha spp., Tipula spp.)
·
Tanymecus
dilaticollis (Maize leaf weevils)
·
Oscinella frit (Frit fly)
·
Rhopalosiphum
maidis (Aphids)
·
Helicoverpa armigera (Cotton bollworm)
·
Diabrotica
virgifera virgifera (American corn rootworm)
Soil insects
The
larvae of certain Elateridae (Agriotes spp., wireworms, Figure 8), Melolonthidae (Melolontha spp., white grubs, Figure 9) and Athoinae (Athous spp.)
feed on roots of maize and even on the stem base. Development of wireworms
takes several years; consequently, larvae of different sizes may be present in
the soil when maize is planted. Development of white grubs takes 3-4 years and
is generally synchronized. Damage normally only occurs from the third larval
stage onwards, starting in the year after adult flight.
Adults
attack young seedlings (rarely germinating seeds) and destroy them. They feed
on young leaves from the leaf margin, and most damage occurs before the 4-leaf
stage (BBCH 14). Higher temperatures enhance feeding. Tanymecus dilaticollis (Figure 10) has one generation per year and
overwinters as pupae in the soil.
Figure 10 Tanymecus dilaticollis (Source: Slutsky, 2013)
Figure 11 Oscinella frit larvae in a grass stem (Source: Nastić, 2013)
The
pest occurs in the central, northern, and western parts of the EU. Its damage
is usually sporadic, rarely attacks whole big fields. The Oscinella frit larvae (Figure 11) of the pest can destroy the
productive apex of the plant, so the maize plant may die. This pest is the main
damager of gardens and smaller fields; it rarely causes hard yield losses on
the great fields. The rolled end of the leaves does not open itself. The
youngest leaves are well etiolated and the plant growth stops. Later the whole plant
dies. The symptoms can be confused with hormone-containing herbicides.
Leaf-rolling is a common symptom, but the etiolating of central leaves is
characteristic of the frit fly.
Maize
leaf aphid (Rhopalosiphum maidis, Figure
12) feed on sorghum, maize, small grains, and other grasses. Some year's
populations are numerous on small grain seedlings in October and cause reduced
yields. They also are known vectors of barley yellow dwarf virus. From early
July until fall, colonies of maize leaf aphids can be found on or near tassels
or whorl leaves in most maize fields. Some fields may have up to 50 per cent
plant infestation level, but this is extremely rare. Normal ranges are from
zero to possibly 2 or 3 percent. There are no data to verify that corn leaf aphids
cause barren stalks. However, it is believed that 30 to 40 percent of heavily
infested stalks may be barren. Periods of dry weather seem to favor increases
in aphid numbers and resulting plant damage. Dry conditions add stress to maize
plants and also prevent the development of the fungal pathogens that infect and
kill the aphids. Maize leaf aphids feed by
sucking sap from the upper leaves and tassels. The infested tassels become
covered with a sticky substance called honeydew that drips onto the leaves and
silks. Pollination probably is affected by honeydew covered silks. Heavily
infested leaves and tassels may wilt and turn brown. A few weeks after the
initial infestation, plants will have a black, sooty appearance due to a sooty
fungus that develops and thrives on the honeydew excreted by the aphids.
The European corn borer (Ostrinia nubilalis, Figure 13), which is to be found almost
universally in Europe and America Hungary America
and the corn borer in Eurasia . It is
interesting to note that it was never able to spread in such great proportions
on its original hosts (hemp, hops, millet and sorghum) as it has in maize.
This migrant species causes serious losses
throughout its range. Cobs are invaded, and developing grain is consumed. There
are two to three generations a year. Helicoverpa
armigera (Figure 14) overwinters as pupae in the soil. Adults appear from
May until the end of October. Eggs are laid on plants at or near flowering. The
principal host on which eggs are laid is maize, but other plants are also
concerned, e.g. tomatoes, weeds, and, in certain areas, cotton. The feeding
larvae can be seen on the surface of plants, but they are often hidden within
plant organs. Bore holes may be visible, but otherwise it is necessary to cut
open the plant to detect the pest. Secondary infections are common.
Figure 14 Helicoverpa armigera
(Source: Szőke, 2013)
Figure
15. Damaged of Diabrotica virgifera virgifera larvae and imago (Source: Szőke,
2012)